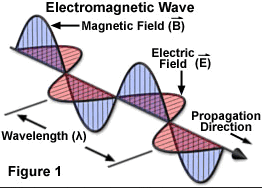
Electromagnetic radiation (EM radiation or EMR) is a fundamental phenomenon of electromagnetism, behaving as waves and also as particles called photons which travel through space carrying radiant energy. In a vacuum, it propagates at the speed of light, normally in straight lines. EMR is emitted and absorbed by charged particles. As an electromagnetic wave, it has both electric and magnetic field components, which synchronously oscillate perpendicular to each other and perpendicular to the direction of energy and wave propagation.
In classical physics, EMR is produced when charged particles are accelerated by forces acting on them. Electrons are responsible for emission of most EMR because they have low mass, and therefore are easily accelerated by a variety of mechanisms. Quantum processes can also produce EMR, such as when atomic nuclei undergo gamma decay, and processes such as neutral pion decay.
EMR carries energy—sometimes called radiant energy—through space continuously away from the source (this is not true of the near-field part of the EM field). EMR also carries both momentum and angular momentum. These properties may all be imparted to matter with which it interacts. When created, EMR is produced from other types of energy and it is converted to other types of energy when it is destroyed.
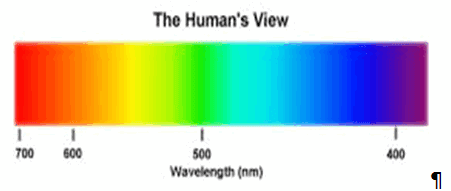
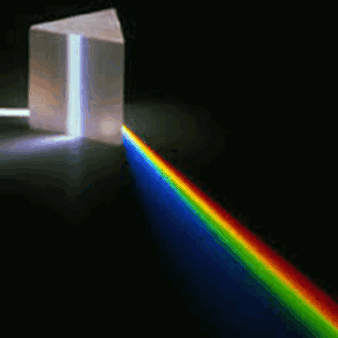
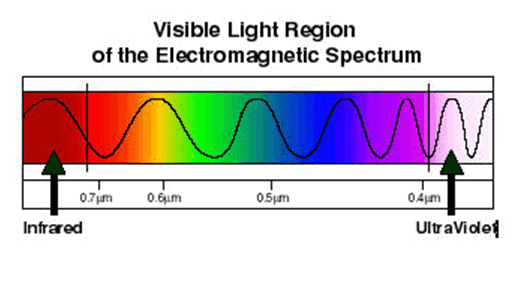
The electromagnetic spectrum, in order of increasing frequency and decreasing wavelength, can be divided, for practical engineering purposes, into radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. The eyes of various organisms sense a relatively small range of frequencies of EMR near and including the visible spectrum or light. Visible light is that part of the spectrum to which human eyes respond. Higher frequencies (shorter wavelengths) have more energy in the photons, according to the well-known law E=hν, where E is the energy per photon, ν is the frequency carried by the photon, and h is Planck's constant. A single gamma ray photon carries far more energy than a single photon of visible light.
In classical physics, EMR is produced when charged particles are accelerated by forces acting on them. Electrons are responsible for emission of most EMR because they have low mass, and therefore are easily accelerated by a variety of mechanisms. Quantum processes can also produce EMR, such as when atomic nuclei undergo gamma decay, and processes such as neutral pion decay.
EMR carries energy—sometimes called radiant energy—through space continuously away from the source (this is not true of the near-field part of the EM field). EMR also carries both momentum and angular momentum. These properties may all be imparted to matter with which it interacts. When created, EMR is produced from other types of energy and it is converted to other types of energy when it is destroyed.
The electromagnetic spectrum, in order of increasing frequency and decreasing wavelength, can be divided, for practical engineering purposes, into radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays and gamma rays. The eyes of various organisms sense a relatively small range of frequencies of EMR near and including the visible spectrum or light. Visible light is that part of the spectrum to which human eyes respond. Higher frequencies (shorter wavelengths) have more energy in the photons, according to the well-known law E=hν, where E is the energy per photon, ν is the frequency carried by the photon, and h is Planck's constant. A single gamma ray photon carries far more energy than a single photon of visible light.















0 comments:
Post a Comment